eCOA and IRT Case Studies: YPrime in Action
These clinical trial case studies highlight how YPrime partners with sponsors and CROs to address protocol complexity, global scale, and regulatory demands—using eCOA, IRT, and proactive oversight to protect data quality and trial timelines.
eCOA Case Study: How YPrime Preserved Data Quality During a Phase 3 Oncology Protocol Amendment
YPrime eCOA for Clinical Trials: A Pediatric Migraine Study
YPrime eCOA in Action: AI-Powered Clinical Trial Localization
eCOA, eConsent and IRT Fact Sheets
YPrime’s fact sheets provide a concise overview of our clinical trial technology capabilities—from advanced eCOA oversight to real-time, audit-ready reporting. These resources are designed to support evaluation, procurement, and study planning conversations.
YPrime Advanced eCOA Oversight
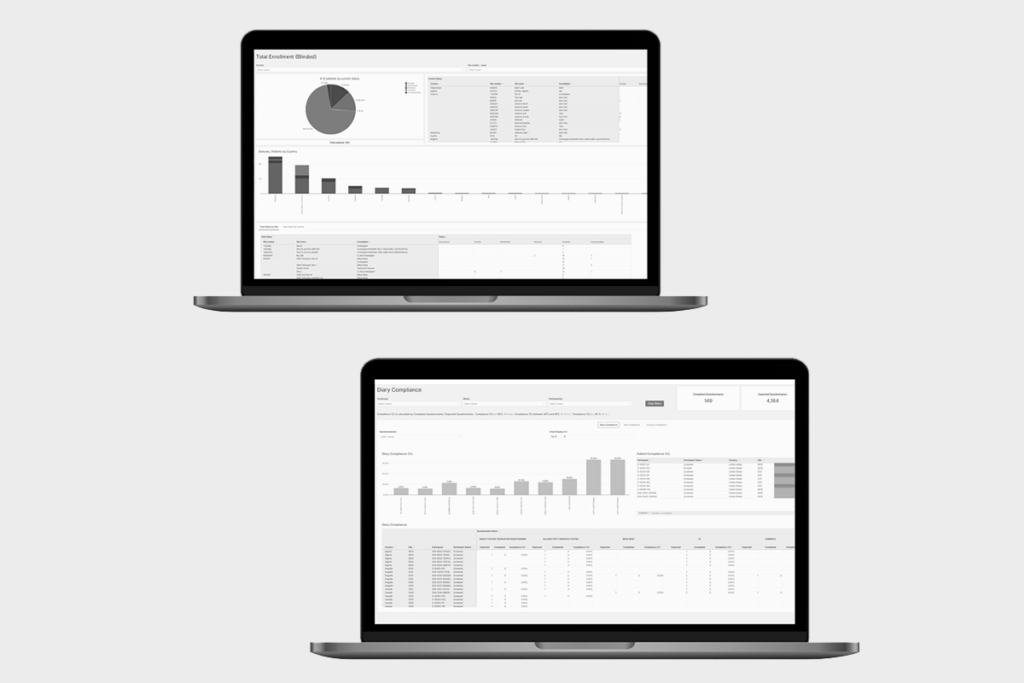
The YPrime Data Suite. eCOA Reporting Improves Decision Making.
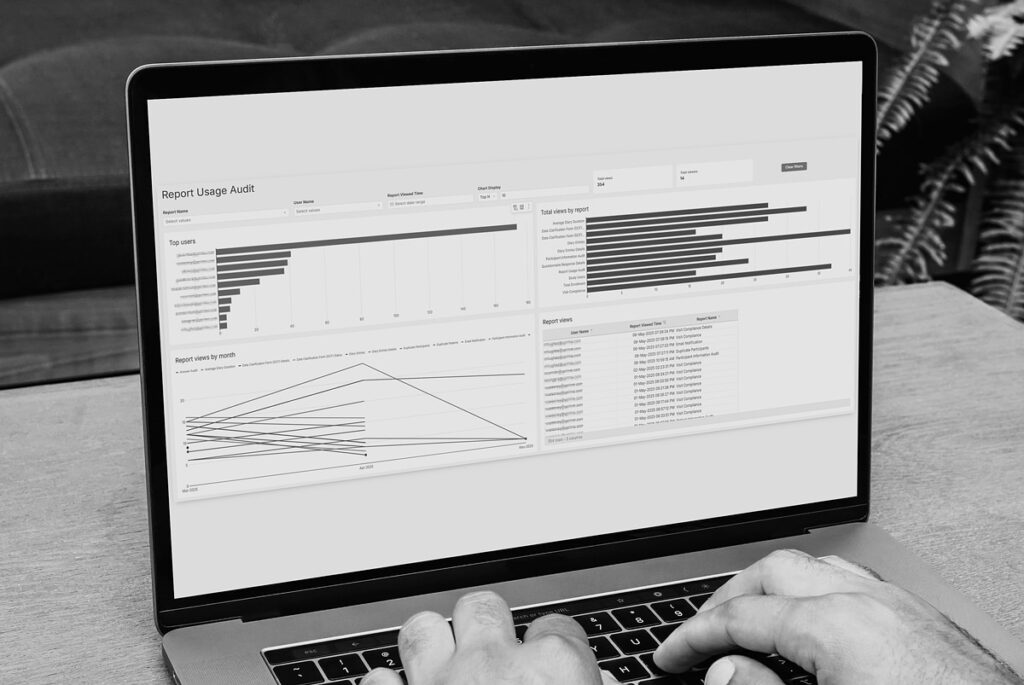
Audit-Ready eCOA Reporting to Improve Clinical Trial Decisions.
We’ve got you covered with strategies and solutions to improve your clinical trial technology, reduce clinical research site burden, and transform your patient experience.
Let’s get started today!
Why Sponsors and CROs Choose YPrime
YPrime’s clinical trial technology is built to support certainty in execution—not just implementation. Across eCOA, IRT, and reporting, our solutions help teams:
- Accelerate study startup and global deployment
- Reduce protocol amendment risk and operational friction
- Maintain consistent, inspection-ready data
- Improve collaboration across sponsors, CROs, and sites
- These outcomes are reflected throughout the case studies and resources on this page.