Regulatory acceptance of data generated by electronic Clinical Outcome Assessment (eCOA) platforms continues to accelerate, creating a clear advantage for clinical trial sponsors seeking faster approvals and shorter time-to-market. As clinical research becomes more complex and competitive, the benefits of eCOA in clinical trials have become increasingly central to how sponsors manage data quality, participant engagement, and operational efficiency.
Across clinical studies, eCOA delivers measurable benefits, including improved data accuracy, stronger participant compliance, increased site productivity, and more streamlined trial workflows. While early adoption introduced challenges, advances in platform design, usability, and training have addressed many of these concerns—driving continued growth in eCOA adoption across therapeutic areas.
Why eCOA Matters in Complex Clinical Trials
As protocols grow more sophisticated, clinical teams are under increasing pressure to monitor patient progress closely, make timely decisions, and respond rapidly to symptom reports that may require adverse event evaluation. These demands place a significant burden on sites and sponsors alike. eCOA plays a central role in addressing these challenges by:
1. Capturing subjective symptoms and patient experiences directly from participants
2. Enabling real-time data collection and remote monitoring
3. Providing immediate access to patient-reported outcome (PRO) data for faster clinical insight
By giving sponsors and clinical trial sites timely, reliable visibility into participant-reported data, eCOA supports better decision-making, faster issue resolution, and more consistent endpoint collection—particularly in complex, global studies.
Insights from Clinical Professionals on eCOA Platforms
To better understand how clinical teams evaluate and use eCOA today, YPrime conducted a survey of clinical professionals across roles and study types. The findings highlight what matters most when selecting and deploying an eCOA platform.
Top Concerns in eCOA Implementation
- Timelines and data quality: The top concerns that repeatedly surfaced in the survey results include timelines/delays, data quality, data change capabilities, protocol amendments, tech flexibility, and technology ease-of-use.
- Measures of success: Survey respondents site visit compliance, diary/scale compliance, and patient enrollment status as the top indicators of endpoint collection success.
- Integration with other technologies: Use of wearables and sensors in clinical research is increasing, and clinical professionals expect them, as well as other eClinical systems, to integrate with their eCOA platform for greater insights.
- eCOA provider relationship: The clinical operations team primarily drives the selection of eCOA providers, with data quality, user interface/ease-of-use, and faster study start-up being important considerations. The burden of switching eCOA providers includes establishing new third-party integrations, procurement qualification of the new provider, and the loss of customizations with the incumbent eCOA provider.
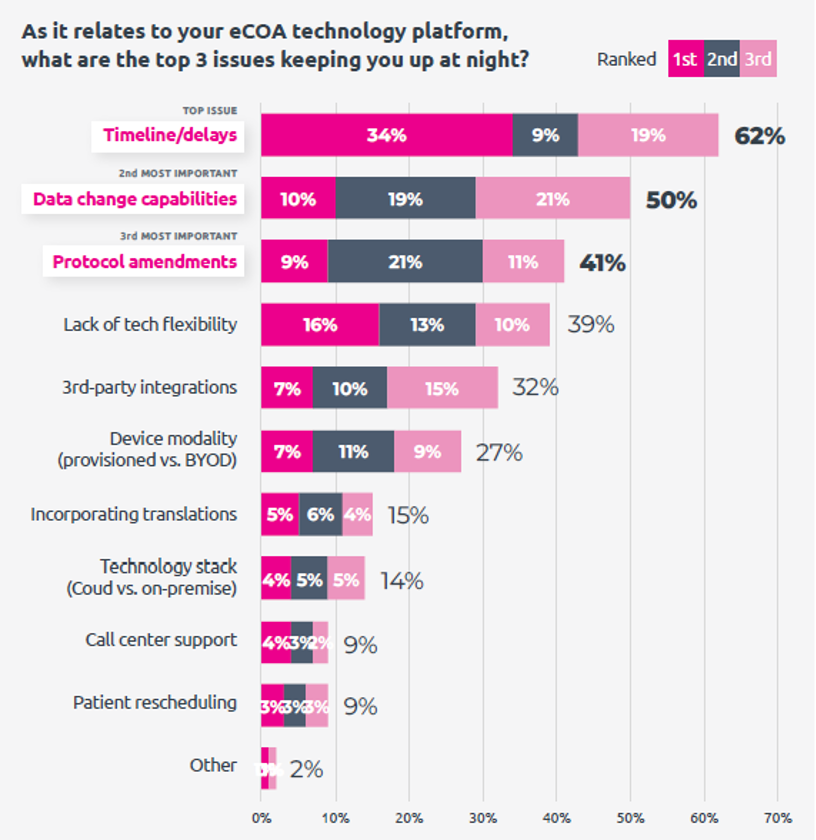
What These Findings Mean for eCOA Selection
The benefits of eCOA in clinical trials are only fully realized when the platform is designed around real operational workflows—not just data capture. Clinical operations leaders are looking for solutions that reduce risk during protocol amendments, support faster, more predictable study startup, provide real-time visibility without increasing site burden, and scale across therapeutic areas and global trial footprint.
A More Strategic Approach to eCOA
At YPrime, we ground our eCOA platform strategy in real-world research, clinical operations feedback, and regulatory expectations. But more importantly, we design eCOA solutions to perform under the realities of live trials—where protocols evolve, timelines shift, and data quality cannot be compromised. Our approach to eCOA focuses on:
- Reducing operational risk through configurable, inspection-ready workflows
- Improving execution by enabling faster decisions and proactive oversight
- Supporting confident, defensible decision-making for sponsors, CROs, and sites
At YPrime, we deliver eCOA that not only delivers measurable benefits in clinical trials, but gives clinical operations leaders the confidence to execute complex studies with greater control, predictability, and certainty.
