Electronic Clinical Outcome Assessments (eCOA) are a core component of modern clinical trials, supporting higher data quality, stronger compliance, and scalable global execution. As studies grow more complex, spanning multiple regions, languages, and digital tools, clinical trial sponsors and CROs must understand not only what eCOA is, but how to approach choosing an eCOA platform that can support real-world trial operations, oversight, and long-term success. This Q&A guide addresses some common questions teams ask when evaluating eCOA for clinical trials, with a focus on data, oversight, globalization, and long-term study success.
Key Considerations When Choosing an eCOA Platform for Clinical Trials
eCOA, or electronic Clinical Outcome Assessment, refers to the digital collection of clinical outcomes data that was historically captured on paper. This includes patient-reported outcomes (PROs), clinician-reported outcomes (ClinROs), observer-reported outcomes (ObsROs), and performance outcomes (PerfOs). eCOA platforms replace manual workflows with validated electronic systems that improve accuracy, traceability, and efficiency throughout the trial lifecycle.
Paper-based assessments introduce transcription errors, delayed visibility, and compliance challenges that are increasingly incompatible with today’s trial designs. eCOA enables real-time data capture, automated validation, and centralized oversight—capabilities that are critical for managing complex protocols, decentralized elements, and global study footprints.
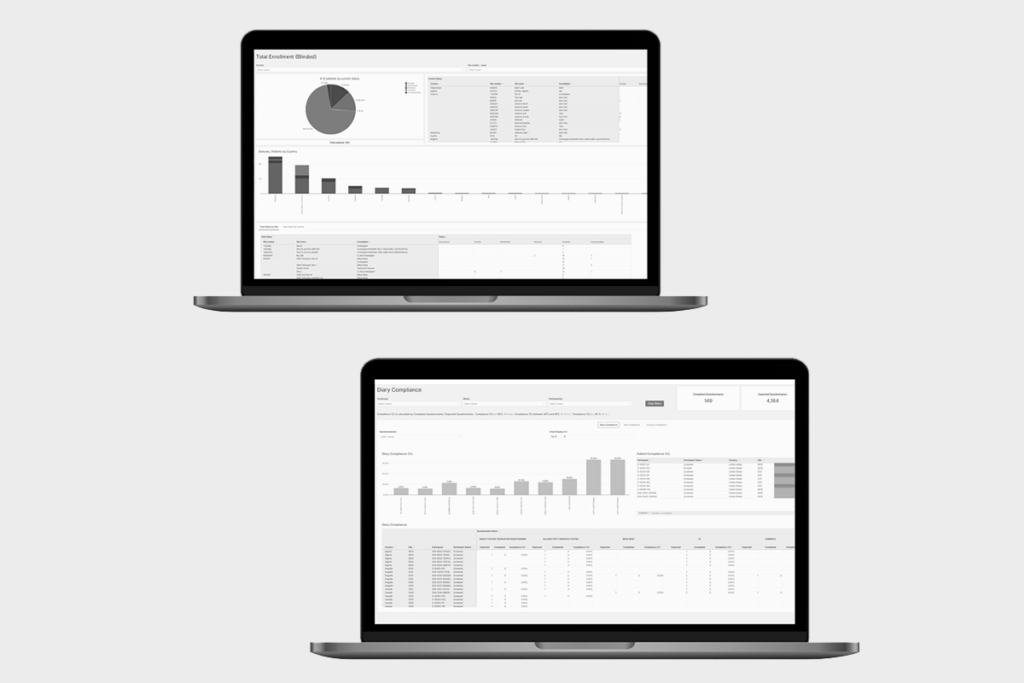
eCOA platforms improve data quality by enforcing completion rules, preventing out-of-window entries, and maintaining full audit trails for every data point. Real-time dashboards allow study teams to identify missing data, protocol deviations, or trends early, reducing downstream data cleaning and supporting inspection readiness.
Participant-facing eCOA tools provide reminders, visit guidance, and intuitive interfaces that make assessments easier to complete correctly and on time. Multilingual support and accessible design reduce burden for diverse populations, while real-time monitoring allows sites to intervene proactively when compliance drops.
eCOA localization ensures that assessments are accurately translated, formatted, and migrated across languages and regions while preserving conceptual equivalence. Errors introduced during localization can delay startup, increase review cycles, and compromise data consistency, making localization quality a critical factor in global trial success.
AI can meaningfully enhance eCOA localization by automating rules-based tasks such as questionnaire mapping and syntax validation. When paired with human linguistic and regulatory review, AI reduces migration errors, shortens approval cycles, and improves consistency across languages without compromising quality or compliance.
Advanced eCOA platforms can integrate with connected devices such as glucometers, blood pressure monitors, wearables, and activity trackers. Device data flows directly into eCOA workflows, enabling continuous, objective measurement alongside patient-reported assessments, reducing reliance on recall-based reporting or frequent site visits.
Integrating connected devices with eCOA improves data completeness, reduces recall bias, and lowers participant burden by automating data capture. Real-time monitoring supports earlier intervention, stronger compliance, and more reliable endpoint analysis, particularly in decentralized, cardiometabolic, CNS, and rare disease trials.
Effective eCOA for clinical trials depends not only on how data is collected, but on how quickly teams can access, interpret, and act on it. Delays caused by fragmented systems, manual exports, or downstream data preparation can limit visibility and slow decision-making across the study lifecycle.
YPrime’s Data Suite addresses this challenge by combining high-performance eCOA and IRT reporting with near real-time data integration. From study startup through closeout, sponsors and CROs gain continuous insight across sites, subjects, devices, and workflows—without waiting for static reports or reconciled files. Dashboards update in near real time, with every data point logged, traceable, and inspection-ready to support faster, more confident decisions.
As trials increase in complexity, timely access to eCOA and IRT data becomes critical. Near real-time insight supports therapeutic-area-specific monitoring needs, such as tracking C-SSRS scores and adverse events in oncology trials, identifying shifts in glucose data in cardiometabolic and GLP-1 studies, or auditing clinician-administered assessments in CNS research. By unifying reporting and data access, sponsors can address risk earlier, maintain protocol compliance, and improve site performance without adding operational burden.
Yes, eCOA is especially well suited for rare disease clinical trials, where small patient populations, global recruitment, and caregiver involvement demand precision and flexibility. Advanced eCOA platforms support adaptive scheduling, offline data capture, observer-reported outcomes, and real-time compliance monitoring, helping ensure that every data point is captured accurately when each participant represents a meaningful portion of the study population. While rare disease trials highlight eCOA’s value most clearly, these capabilities extend broadly across therapeutic areas. eCOA is widely used across therapeutic areas including oncology, cardiometabolic and metabolic disease, CNS, hematology, infectious disease, and many others.
Common risks include late localization, insufficient migration testing, unclear approval of workflows, and limited visibility into compliance. These challenges can be mitigated by selecting an eCOA platform with configurable architecture, structured change control, and experienced global delivery teams.
Future-ready eCOA platforms combine configurable workflows, AI-assisted quality controls, seamless system integration, and globally informed user experience design. These capabilities allow sponsors to adapt to evolving protocols, regulatory expectations, and digital trial strategies without disrupting execution.
Choosing an eCOA platform is not simply a technology decision—it directly impacts data quality, timelines, regulatory readiness, and participant experience across the entire study lifecycle. A thoughtful eCOA strategy helps sponsors and CROs execute with greater confidence, consistency, and control.
YPrime’s eCOA platform is built to support modern trial demands, combining configurable workflows, AI-supported localization, integrated data access, and global delivery expertise. For sponsors and CROs navigating increasingly complex studies, YPrime provides the structure, visibility, and flexibility needed to execute eCOA for clinical trials with confidence. To learn more, visit www.yprime.com/ecoa
Check Out What Our Experts Have to Say
about trial design, data capture, operational efficiencies, and, ultimately, how to solve with certainty in clinical research.