Our IRT Platform
Solves Real Clinical Trial Challenges.
62% say trial
delays are
the #1 issue
With their IRT platform.2
65% say protocol amendments
take ~6 weeks
To implement.2
88% say data
quality is the
#1 attribute
When choosing an IRT platform.2
Clinical trial teams consistently cite IRT as a source of delay and risk when systems cannot adapt to protocol change. YPrime IRT is purpose-built to address these challenges by aligning flexibility, expert governance, and execution quality.
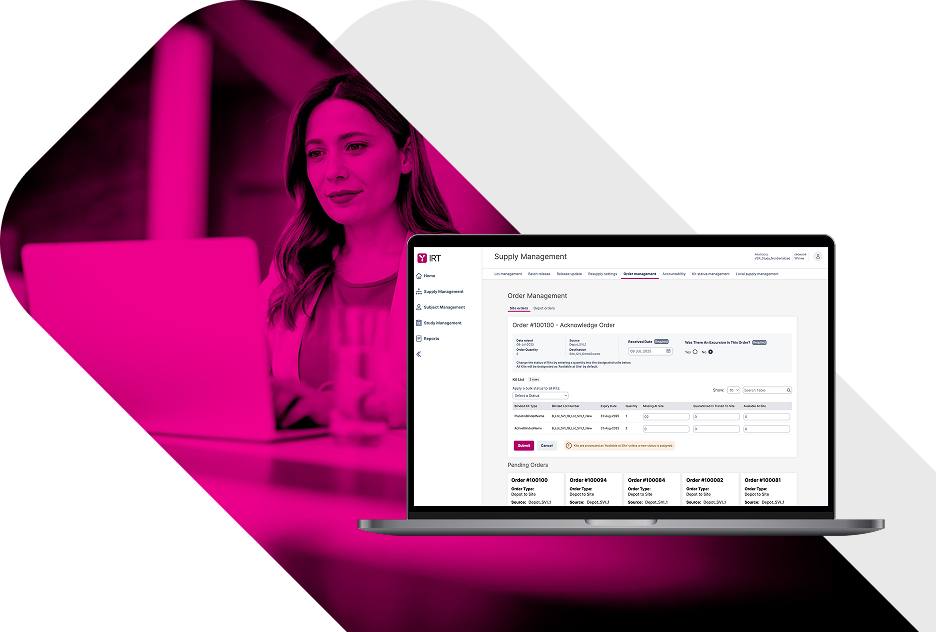
Flexible IRT with Expert Governance.
Clinical trial IRT systems must balance adaptability with control. YPrime’s IRT platform supports operational updates efficiently, while applying appropriate oversight to changes that impact protocol logic, supply strategy, or system behavior.
Industry-Leading Quality in IRT for Clinical Trials.
Quality is foundational to successful clinical trials. YPrime’s IRT platform provides transparent access to clinical trial data throughout the study lifecycle, enabling proactive oversight rather than reactive remediation.
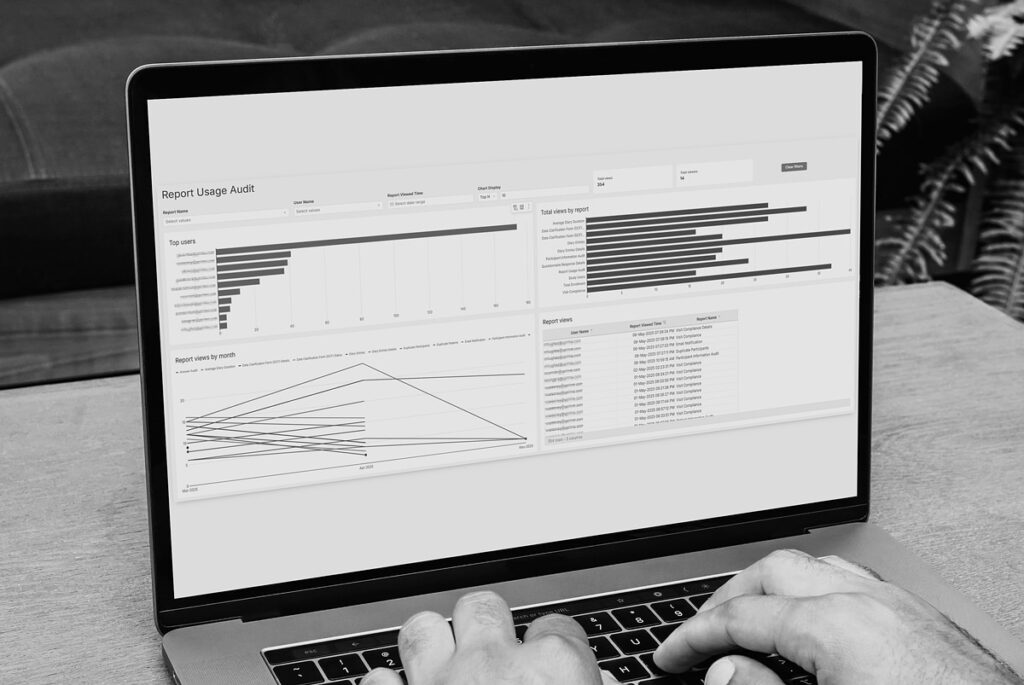
IRT Data Monitoring that Improves Decision-Making.
YPrime IRT delivers the real-world visibility sponsors and CROs need to manage enrollment, supply, and risk proactively.
Our platform helps you:
IRT for Clinical Trials—Designed for Execution at Scale.
50%
faster
IRT startup times.
97.6%
SLA
Ticket SLA achieved.
26
second
In-house help desk speed to answer
YPrime IRT supports complex, global trials while maintaining control, compliance, and data integrity.
YPrime has proven to be quite flexible in setting up solutions but also adjusting midway, being able to change back-end programming to adjust and accommodate earlier stage changes.
IRT for Clinical Trials Backed
by Experience That Matters.
YPrime supports sponsors and CROs with clinical trial technology and expertise that reduce site burden, improve execution, and protect data quality—without sacrificing compliance. Let’s get started today!
Explore Expert Insights on IRT for Clinical Trials.
Continue learning how sponsors evaluate, implement, and optimize IRT in complex studies.