AT YPRIME, WE
Increase
speed
to accelerate clinical trial timelines
Empower
flexibility
to adapt to your most complex trials
Inspire
certainty
to empower you to make informed decisions
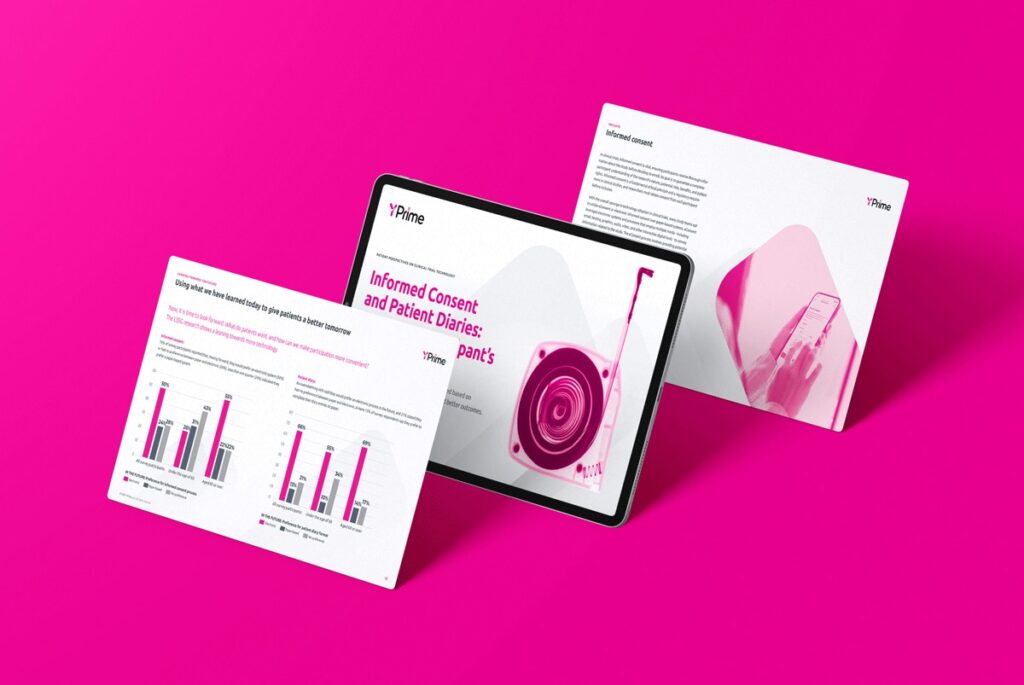
50%
faster
IRT startup times
4X
swifter
protocol amendments
100%
configurable
with pre-validated code
No other data science team at any other eCOA company helps construct data standards for my studies.
Let’s talk.
We’ve got you covered with strategies and solutions to improve your clinical trial operations, reduce site burden, and transform your patient experience.
Let’s get started today!
Looking for ideas and inspiration
to improve your clinical trials?
Access our white papers, case studies, and blogs to discover new ways to solve for certainty in clinical trials.