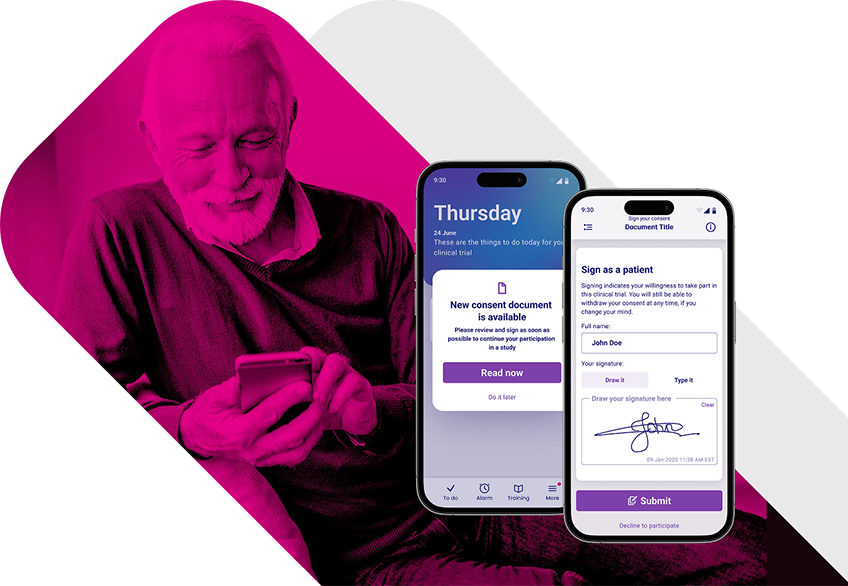
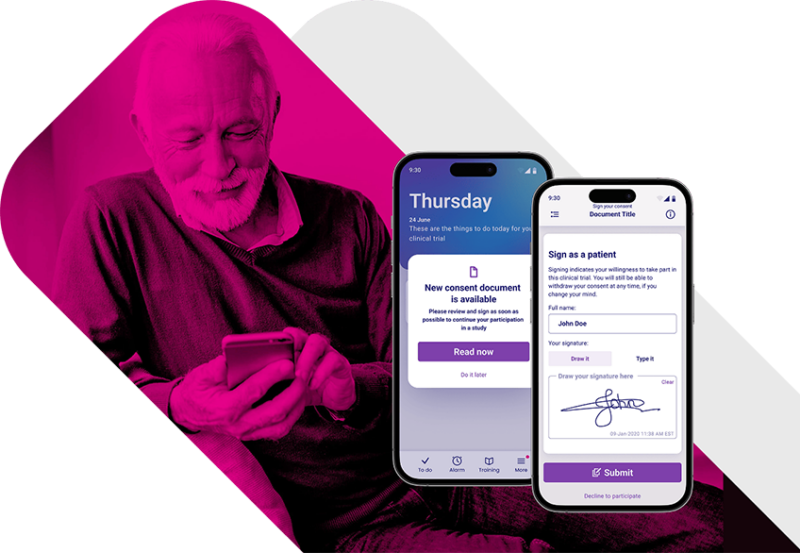
Simplify Clinical Trial Enrollment with an Advanced eConsent Platform.
Present
dynamic trial
information
Deliver interactive, multimedia consent content that clearly explains trial requirements, expectations, and risks—supporting truly informed consent.
Support your
patients on
any device
Enable participants to review and complete electronic consent on mobile, tablet, or desktop devices, in their own language and at their own pace.
Deliver
immediate,
built-in support
Provide real-time access to assistance during the consent process, reducing delays, minimizing site workload, and preventing incomplete or incorrect submissions.
Enhance Clinical Trial Integrity with Secure, Compliant eConsent.
Regulatory requirements for electronic consent continue to evolve across regions. YPrime’s eConsent platform is designed to support compliant, ethical informed consent across global clinical trials, helping sponsors and CROs remain inspection-ready at every phase of the study.


Re-Consent Without Disruption.
Paper consent and manual re-consent workflows slow trials down, especially when protocols change. Missed signatures, outdated versions, and manual tracking introduce unnecessary risk for sites and sponsors.
YPrime eConsent transforms re-consent into a seamless digital workflow. New consent versions are deployed instantly while outdated forms are automatically retired. Only impacted participants are flagged for re-consent, allowing sites to focus effort where it matters most. Progress is tracked in real time with complete audit trails, and participants can clearly see what has changed, review updates, and re-sign from any device, anywhere.
The result is faster, smarter consent and re-consent management, without confusion, compliance gaps, or operational disruption across global clinical trials.
At YPrime, data security is at our core. All data in our platform is encrypted, and every version change is stored for future compliance needs or audits.
Solve Enrollment Challenges
with Certainty.
When we work together, you gain confidence that your clinical trial consent process is compliant, efficient, and designed for both patients and sites. Let’s simplify enrollment, and start trials stronger.
Explore Insights from Our Experts.
Gain valuable perspectives on clinical trial consent, participant engagement, regulatory readiness, and operational execution—so you can run your clinical trials with certainty.
Sources:
1Considerations For Improving Patient Recruitment Into Clinical Trials