YPrime IRT (RTSM): Built for Flexibility, Trusted for Quality
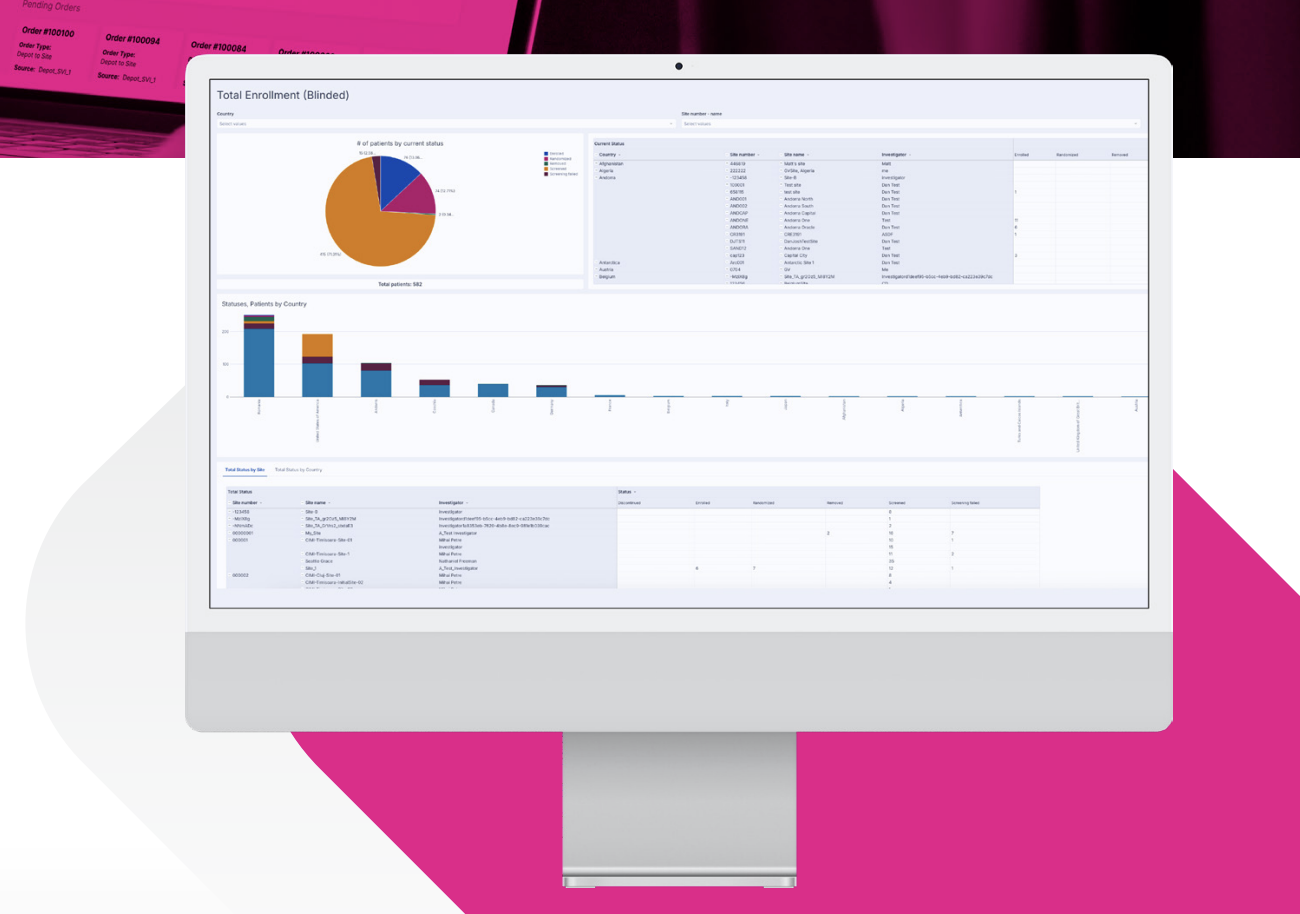
Today’s trials evolve faster than ever. Protocols change, timelines shrink, and complexity increases across every stage. YPrime IRT (RTSM) helps sponsors and CROs deploy faster and adapt confidently, with real-time visibility across subjects, sites, and supplies. Delivering unmatched flexibility, speed, and quality, YPrime IRT keeps trials moving forward without compromise.
Why Sponsors Choose YPrime IRT
Every clinical trial has individual challenges, and the ability to adapt quickly is the difference between meeting timelines and facing delays. YPrime IRT empowers sponsors to handle real-world protocol changes without delays or costly revalidation. It supports adaptive designs, extension studies, and complex visit logic—with simple changes turned around in days.
Ready to See YPrime IRT in Action?
From FPI to last-patient-last-visit, YPrime gives you the flexibility, speed, and quality to keep your trials moving forward. Complete the form to view the fact sheet or book a personalized demo.