As the GLP-1 receptor agonist market advances toward a projected $157.5 billion by 2035,¹ pharmaceutical companies are adapting to the evolving demands of this competitive field. With over 300 GLP-1 drugs in active development,² companies seek more efficient approaches to accelerate trial completion while maintaining quality standards—making GLP-1 trial innovation an essential differentiator in this crowded landscape.
The GLP-1 market is rapidly expanding beyond its initial focus on weight management and diabetes. Researchers are now exploring various potential applications, from cardiovascular disease prevention and addiction treatment to sleep apnea management and liver health. Emerging research also suggests possible neuroprotective effects in Alzheimer’s and Parkinson’s disease, as well as benefits in managing polycystic ovary syndrome (PCOS). This broadening scope of GLP-1 therapies underscores the need for adaptable clinical approaches where GLP-1 trial innovation can support success across new indications.
GLP-1 research has prompted clinical operations groups to adapt their approaches for greater efficiency, with a variety of technologies used in tandem throughout their studies. As this medication class expands, organizations partnering with technology providers like YPrime—whose innovative eCOA platform and flexible product development approach can be tailored to meet specific sponsor requirements—are better positioned to accelerate their development programs while maintaining high-quality standards.
The remarkable outcomes in weight management studies have set the bar high for GLP-1 research across indications. At YPrime, we learned that operational efficiency becomes especially critical when managing large-scale weight loss studies. This was the case when we supported a recent GLP-1 study with 10,000+ patients internationally with our eCOA platform. We’ve implemented strategic planning and innovative approaches that help programs stay on track and maximize their potential. These approaches are core to our philosophy on GLP-1 trial innovation and can be extended to other studies and indications.
“Evaluating GLP-1 therapies demands efficient processes and technologies to manage complex data collection across primary, secondary, and exploratory endpoints.”
Some clinical trials must capture a comprehensive range of data, as evidenced by GLP-1 weight management studies, and require robust eCOA functionality. While weight loss may be a primary endpoint, these studies typically assess multiple dimensions and include quality-of-life measurements, laboratory values, mood assessments, and improvements in obesity-related comorbidities such as cardiovascular outcomes, sleep apnea severity, and liver function. This multifaceted approach to evaluating GLP-1 therapies demands efficient processes and technologies to manage complex data collection across primary, secondary, and exploratory endpoints.
Recent trial experiences highlight the critical nature of operational innovation. One global pharmaceutical company, with YPrime’s assistance, had to rapidly implement a bring-your-own-device (BYOD) approach mid-study to prevent patient dropout and data collection issues. While the adaptation proved successful, it underscored the importance of implementing flexible technology. You can read more in YPrime’s GLP-1 playbook here.
STEP-BY-STEP GUIDE
Learn more about leveraging technology in this YPrime Playbook:
Mastering Clinical Trials in the New Era of GLP-1
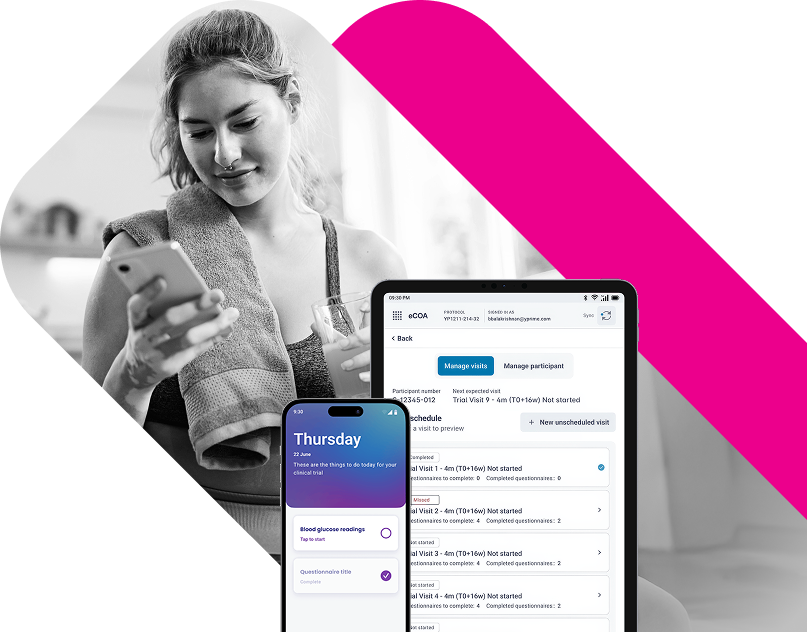
Several operational challenges common to GLP-1 trials require careful attention and technology designed to address them. These include patient retention during extended follow-up periods, collection of complex endpoints, global execution across languages and cultures, and successful technology integration. At YPrime, our role in driving GLP-1 trial innovation helps sponsors address these challenges through fit-for-purpose digital solutions and adaptive strategies.
Operational efficiencies can contribute significantly to trial success. Companies that invest in advanced data collection systems, like the glucose monitoring integration we’ve developed, can help studies run more smoothly and potentially yield higher-quality data. Our teams support these efforts by managing the eCOA components throughout the study lifecycle.
As the GLP-1 market continues to grow, pharmaceutical companies must adapt their clinical trial approaches to maintain competitiveness. Technologies that enable efficient data collection, streamlined processes, and patient-centered approaches can provide valuable advantages. At YPrime, we’re proud to offer the tools and expertise to help navigate these complex trials successfully.
Learn more about how YPrime can help you optimize your GLP-1 clinical trial. Download our playbook, Mastering Clinical Trials in the New Era of GLP-1, or email marketing@yprime.com today!
Sources
1 Roots Analysis Business Research & Consulting, GLP-1 Market: Industry Trends and Global Forecasts. https://www.rootsanalysis.com/reports/glp-1-market.html. Accessed January 27, 2025.
2 Clinical Trials Arena, 2024 was a record year for obesity trials and 2025 is already poised to take over. https://www.clinicaltrialsarena.com/analyst-comment/2024-record-year-obesity-trials-2025-poised-take-over/. Accessed January 27, 2025.
Check Out Our Other eCOA Resources
about trial design, data capture, operational efficiencies, and, ultimately, solving for certainty in clinical research.



