Configurable, Scalable eCOA for Complex Clinical Trial Protocols.
Every study is different. YPrime’s flexible eCOA platform adapts to your protocol, region, and patient population—without slowing you down.
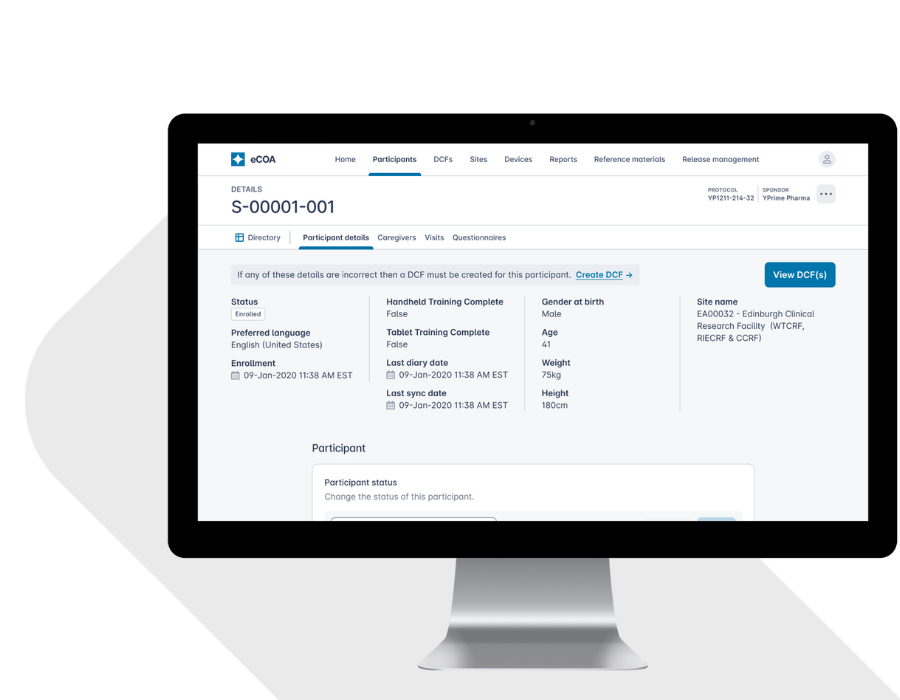
A Patient-Centered Approach Improves Compliance.
Better experiences lead to better data. YPrime’s eCOA app is designed using real-world usability research to support participants across age groups, regions, and therapeutic areas.
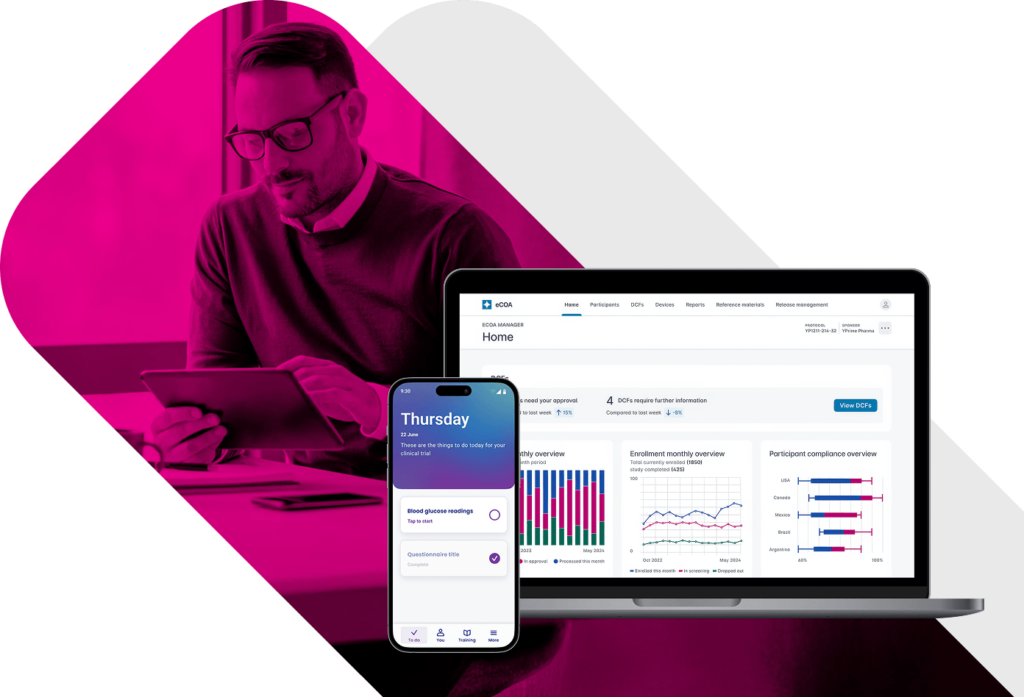
eCOA Reporting
and Analytics for Real-Time Clinical Trial Oversight.
Most eCOA platforms delay insight until it’s too late to act. YPrime’s eCOA reporting delivers near real-time visibility into participant activity, site performance, and data quality—supporting proactive oversight and faster intervention.
Want a preview? Book a demo of our eCOA reporting dashboards.
Future-Ready eCOA—Built for What’s Next in Clinical Trials.
47% faster study startup
100+ countries supported
BYOD and provisioned device ready
YPrime has a clean and seamless UI. It is able to take the data and put it into graphs and figures to show where the data is trending.
Trusted eCOA Partner for Global Clinical Trials
YPrime’s eCOA platform has been deployed in nearly 1,000 clinical trials across 100+ countries and 19 therapeutic areas. Sponsors rely on YPrime for studies requiring rapid startup, mid-study adaptation, and inspection-ready data from first patient in through database lock.
~1,000
Studies Implemented Globally

19+
Therapeutic
Areas

15+
Years eClinical Technology and Services
Future-Ready eCOA for
Your Clinical Trials.
Clinical research is evolving—and your eCOA platform must keep pace. YPrime’s eCOA is built to support new endpoints, connected devices, evolving regulatory expectations, and global scale—without sacrificing data quality or startup speed. See how YPrime can support your next study.
Explore eCOA Insights from Our Experts.
Gain expert perspectives on clinical trial design, eCOA data quality, operational efficiency, and how sponsors solve with certainty in modern clinical research.