Clinical Trial Technology — What Patients Want, and What Sponsors Should Avoid.
Patient-centricity in clinical research is no longer a trend—it is an expectation shaped by evolving regulations, ethical standards, and the growing recognition of the essential role participants play in trial success. As clinical trial technology becomes more embedded in study execution, sponsors face a critical question:
Are the tools we deploy actually working for the participants who use them?
From informed consent to daily diary entries, clinical trial participants interact with technology at some of the most sensitive moments of a study. Understanding patient preferences—and the friction points that undermine trust, compliance, and retention—is essential to designing trials that perform as intended.
This research explores how patients experience clinical trial technology today, including where digital tools succeed, where they fall short, and what sponsors should avoid when implementing technology-driven workflows.
Patient Perspectives on Clinical Trial Technology
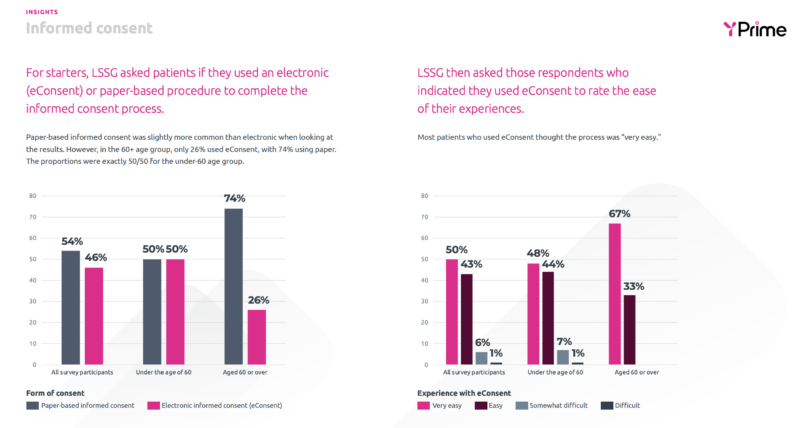
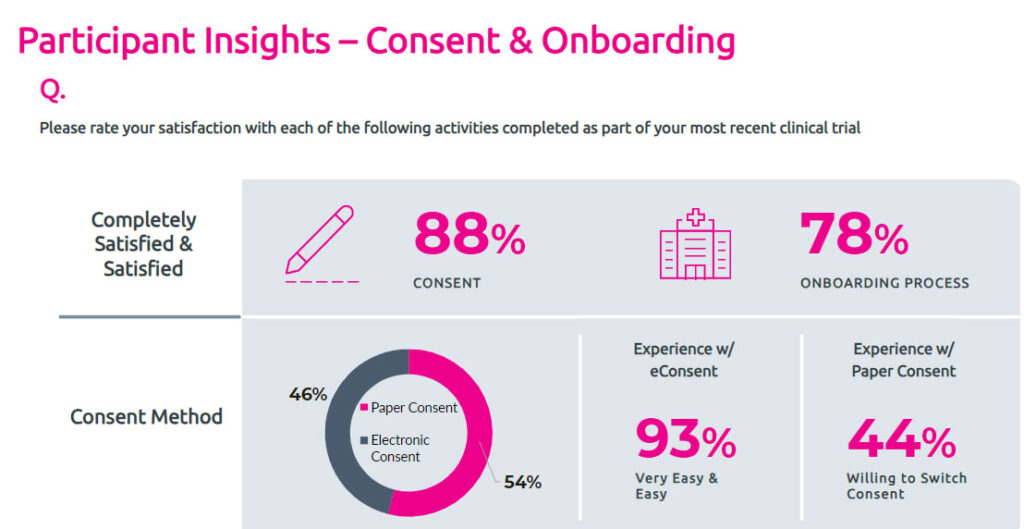
Informed Consent and Patient Diaries:
The Study Participant’s Point of View
Download the white paper to discover:
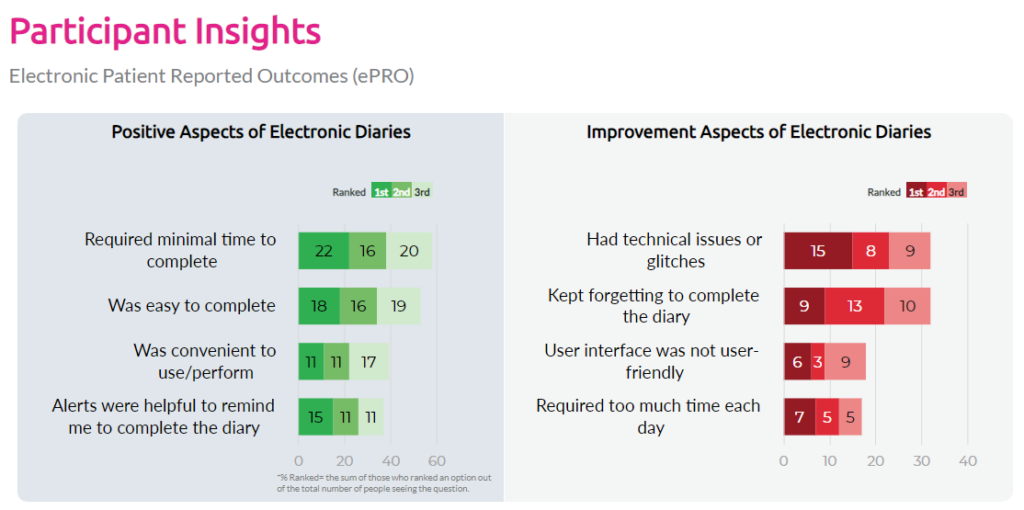
Whether patients prefer electronic diaries (eDiaries) over paper—and why
How clinical trial participants experience eConsent in real-world studies
The most common usability challenges participants encounter
A Glimpse at the Clinical Trial Technology Insights
This report offers practical guidance for sponsors and clinical teams looking to align technology decisions with participant needs—helping reduce burden, improve adherence, and support stronger trial outcomes through more thoughtful technology design.



©2024 Y-Prime, LLC.All Rights Reserved.