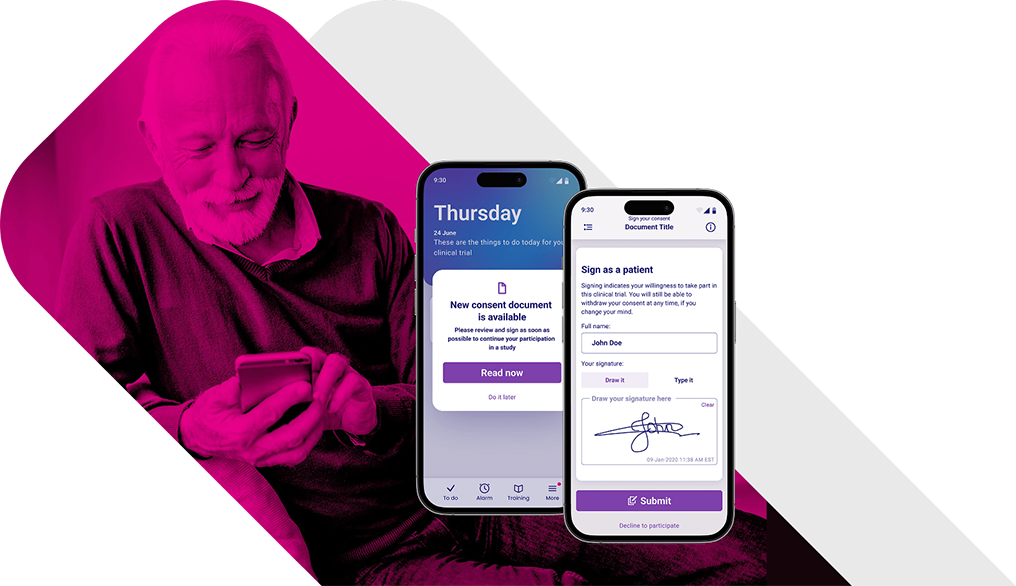
Simplify Your Clinical Trial Enrollment with Our
eConsent Platform.
Present
dynamic
information
That clearly explains your trial.
Support your
patients on
any device
In their own language and at their own pace.
Deliver
immediate,
built-in support
When your patients need it most.
Let Our Clinical Technology
Platform Simplify
Your Trial Experience.


Enhance Clinical Trial Integrity with YPrime’s eConsent Solution.
Navigating regulatory requirements is daunting. At YPrime, we ensure electronic consent is obtained legally and ethically, regardless of location, providing certainty in the integrity of your clinical trial data.
At YPrime, data security is at our core. All data in our platform is encrypted, and every version change is stored for future compliance needs or audits.
Let’s Solve with Certainty in Clinical Research—Together!
When we work together, you know that your clinical trial data is always in compliance and that the process will be easy. Let’s get started!
Explore Insights from Our Experts.
Gain valuable perspectives on clinical trial design, high-quality data capture, operational efficiencies, and, ultimately, how to solve with certainty in clinical research.
Sources:
1Considerations For Improving Patient Recruitment Into Clinical Trials