Top 3 issues keeping
clinical leaders up at night…
62% say timeline/delays
are the #1 issue
Most studies are behind schedule1
50% say data-change capabilities are the
#2 problem
They need high-fidelity data1
41% say protocol amendments are the #3 concern
Multiple protocol amendments impact timelines1
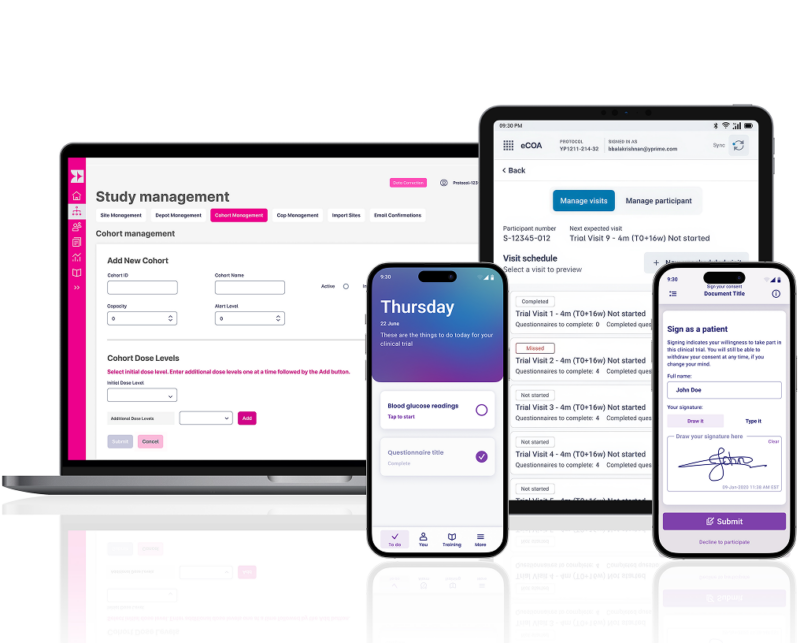
YPrime’s clinical trial technology platform offers patient engagement features across eConsent and eCOA, leveraging user-friendly design, personalization, and behavioral science to drive patient retention and adherence.
Experience That Matters.
~1,000
Studies Implemented
Globally
19+
Therapeutic
Areas
14+
Years eClinical Technology
and Services
We’ve got you covered with strategies and solutions to improve your clinical trial technology, reduce clinical research site burden, and transform your patient experience.
Let’s get started today!
Explore Insights from Our Experts.
Gain valuable perspectives on clinical trial design, high-quality data capture, operational efficiencies, and, ultimately, how to solve with certainty in clinical research.
Source: