In GLP-1 clinical trials, technology decisions are no longer tactical, they are foundational. When sponsors select trial technologies, they are not simply choosing tools; they are architecting the data infrastructure that determines study quality, execution speed, and regulatory confidence.
The GLP‑1 space exemplifies this shift. With over 300 GLP-1 drugs currently in active development¹ and the global market projected to reach $157.5 billion by 2035,2 the operational stakes have reached new heights. In this environment, connected devices supported by purpose-built eCOA platforms are no longer optional. They are essential infrastructure for data-driven clinical trial execution.
“The question is no longer whether to integrate connected devices into GLP-1 trials, but how to do so in a way that ensures data integrity, operational scalability, and regulatory readiness.
As GLP‑1 research expands far beyond its traditional diabetes and weight management roots, the complexity of data collection has grown exponentially. Today’s GLP‑1 trials are investigating breakthrough applications in sleep apnea management, addiction treatment, cardiovascular disease prevention, non-alcoholic steatohepatitis (NASH), neurodegenerative diseases like Alzheimer’s and Parkinson’s, and PCOS management.
Each indication demands precise, protocol-critical data that manual collection methods simply cannot deliver with the accuracy and timeliness modern trials require.
Why Precision Data Matters with eCOA and GLP-1 Trials
In this competitive environment, data quality isn’t just about regulatory compliance. It’s about competitive advantage. Sponsors need every data point to be accurate, timely, and defensible. The traditional approach of manual data collection, with its inherent risks of transcription errors, delayed reporting, and inconsistent measurements, can no longer meet these demands.
Connected devices have emerged as the solution that enables sponsors to capture real-world, protocol-critical data with unprecedented precision. These technologies eliminate human error while providing the granular, continuous monitoring that today’s sophisticated GLP‑1 studies require.
Connected Devices Across Expanding GLP‑1 Indications
The value of connected devices in GLP-1 trials lies in their adaptability across diverse therapeutic endpoints, when supported by an experienced eCOA platform.
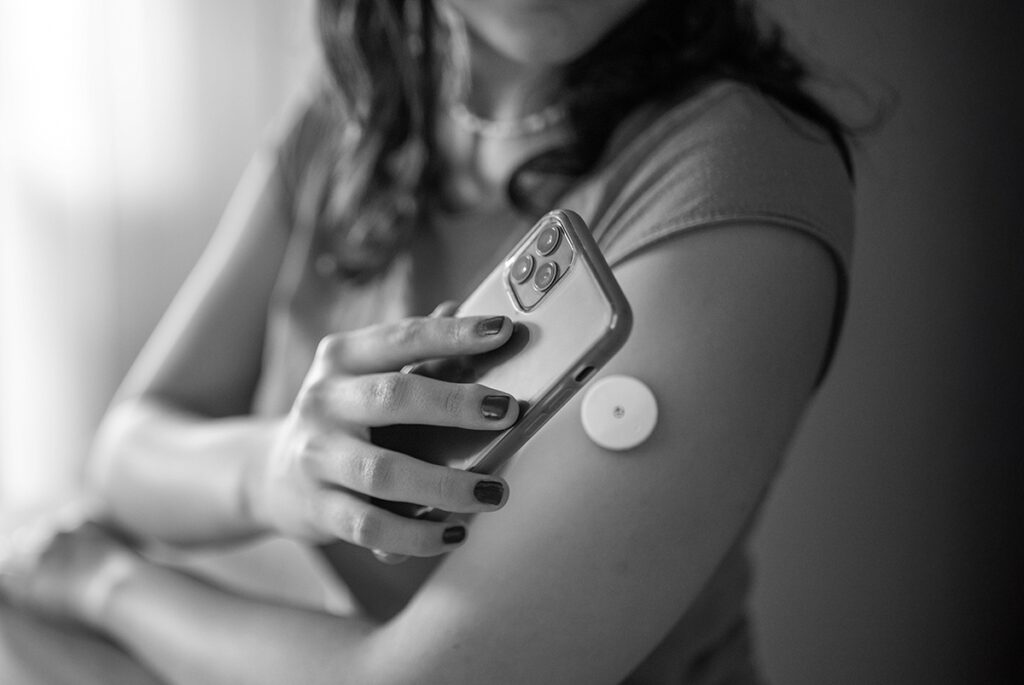
GLP-1 SPOTLIGHT on Connected Glucose Monitoring
Connected glucose monitoring (BGM + CGM) integration transforms reactive monitoring into proactive, data-driven participant management, enabling clinicians to make informed, individualized healthcare decisions throughout the study lifecycle.
Real-Time Clinical Decision Support
Individualized Participant Care
Operational Excellence
The Integration Advantage: Unified eCOA and Device Data
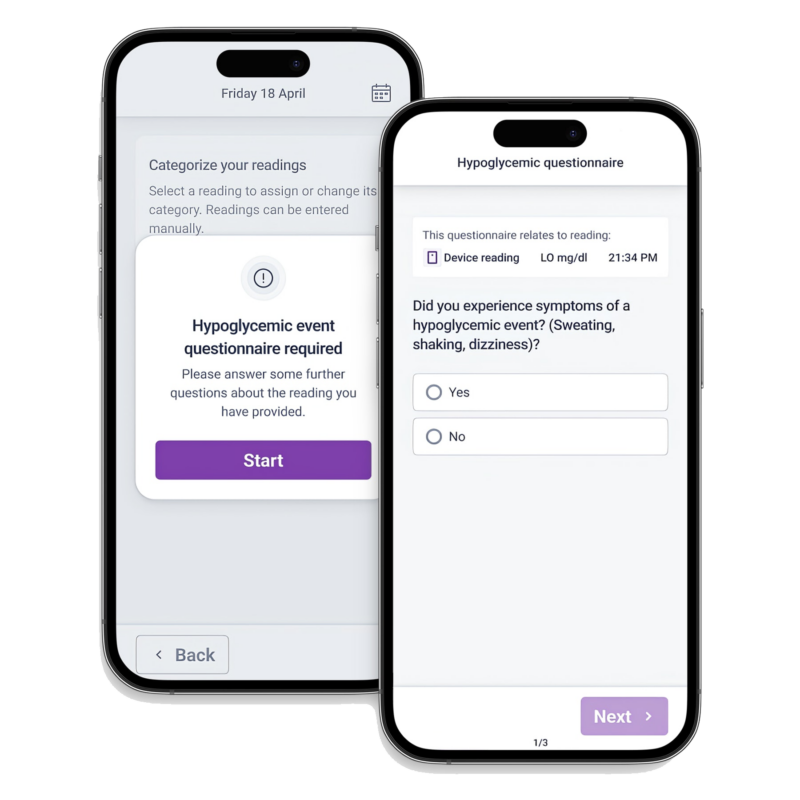
The true value of connected devices is realized only when data flows into a unified eCOA environment. Fragmented device feeds and disconnected dashboards introduce risk, delay, and oversight gaps.
YPrime’s eCOA platform harmonizes device data with participant-reported outcomes, maintains complete audit trails, and supports inspection-ready reporting across global studies, allowing teams to monitor safety, compliance, and protocol adherence in near real time.
Enabling Adaptive, Patient-Centric GLP-1 Trial Designs
Continuous access to high-quality device data enables more adaptive and responsive trial designs. Sponsors can identify emerging risks earlier, optimize dosing strategies, and refine protocols mid-study, capabilities that are especially valuable in fast-moving GLP-1 programs.
At the same time, passive data collection improves participant experience, reducing burden and increasing retention, critical factors in long-duration metabolic studies.
Connected Devices Are Now Core GLP-1 Trial Infrastructure
Connected devices have moved from “nice-to-have” to mission-critical infrastructure in GLP-1 trials. Sponsors that invest in eCOA platforms purpose-built for device integration gain measurable advantages in data quality, execution speed, and operational confidence.
Sources
1 Clinical Trials Arena, 2024 was a record year for obesity trials and 2025 is already poised to take over. https://www.clinicaltrialsarena.com/analyst-comment/2024-record-year-obesity-trials-2025-poised-take-over/. Accessed May 29, 2025.
2 Roots Analysis Business Research & Consulting, GLP-1 Market: Industry Trends and Global Forecasts. https://www.rootsanalysis.com/reports/glp-1-market.html. Accessed May 29, 2025.
Check Out What Our Experts Have to Say
about trial design, data capture, operational efficiencies, and, ultimately, how to solve with certainty in clinical research.