eCOA Joint Assessments—How Purpose-Built eCOA Improves Data Quality in Clinical Trials
Joint inflammation is a core symptom across a range of chronic conditions, including psoriatic arthritis, ankylosing spondylitis, rheumatoid arthritis, and other autoimmune or musculoskeletal diseases. As global prevalence continues to rise, so does the need for targeted therapies supported by high-quality clinical trial data.
Advancing these therapies depends on clinical trials that can consistently and accurately capture joint activity and treatment response across sites, assessors, and regions. Traditional joint assessment methods—often paper-based or adapted from non-digital workflows—introduce variability, training burden, and downstream data cleaning challenges, creating bottlenecks in clinical research.
This YPrime brief explores how purpose-built eCOA joint assessments, designed specifically for clinical trial workflows, help sponsors and CROs improve data consistency, reduce assessment variability, and strengthen endpoint reliability across inflammatory and musculoskeletal studies.
eCOA that ImprovesJoint Assessments in Clinical Trials
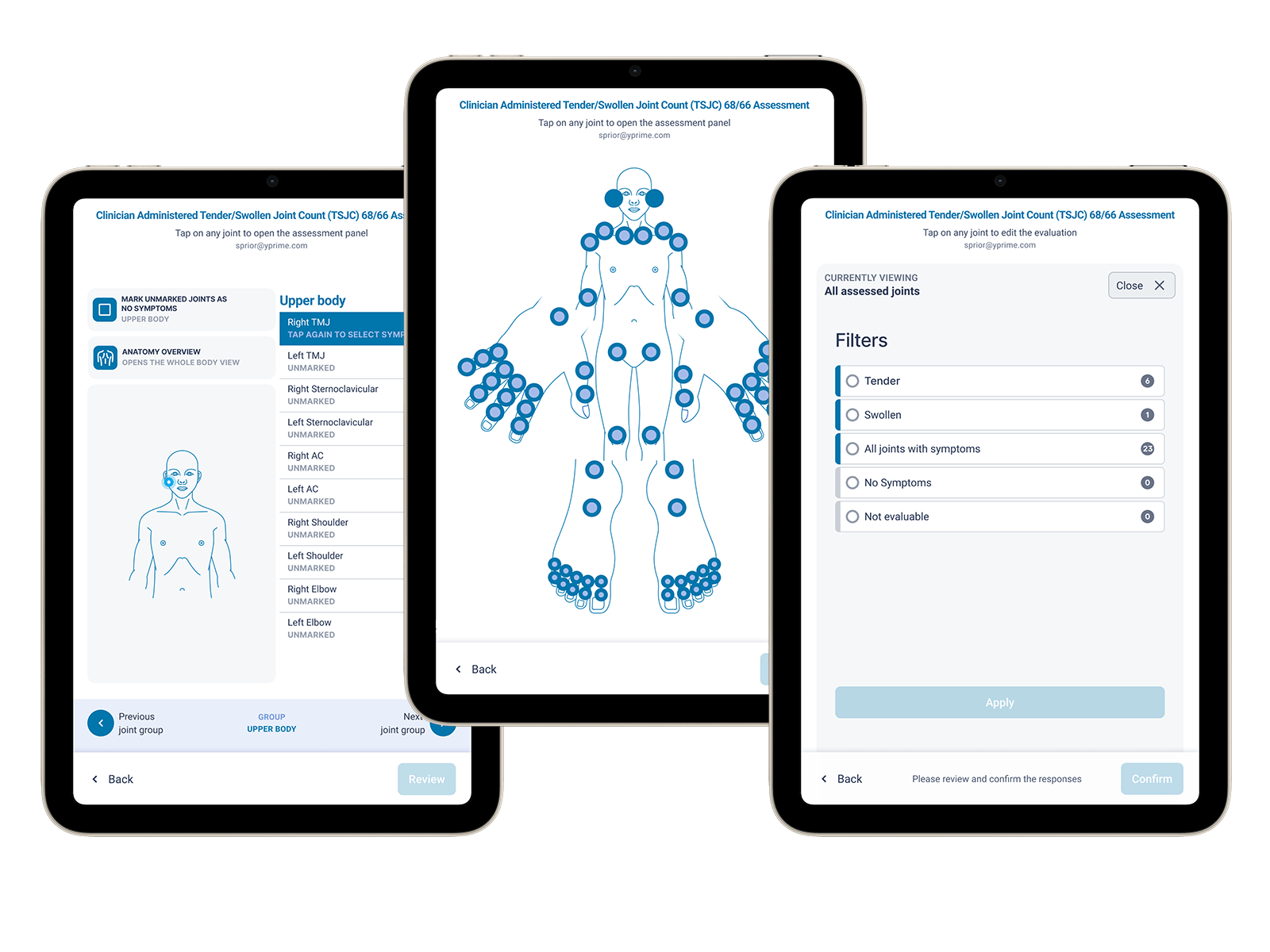
Generic eCOA configurations are often insufficient for complex clinical assessments like joint counts. A new approach to Tender Swollen Joint Count (TSJC) assessments, developed through close collaboration with clinical experts, is helping study teams simplify data capture while improving consistency across sites.
Key eCOA capabilities supporting joint assessments include:
Download the YPrime Tender Swollen Joint eCOA Brief to learn how custom eCOA joint assessments can improve data capture, assessor consistency, and trial execution in inflammatory and musculoskeletal studies.
For additional resources and information on how YPrime can improve your clinical trials, visit our YPrime eCOA page and our Tender Swollen Joint Count (TSJC) eCOA assessment page.