Corporate Responsibility.
At YPrime, ethical clinical research goes beyond compliance—it’s our commitment to a better future.


Security Measures.
Protecting your clinical trial data requires more than just compliance— it demands proactive security at every level. YPrime employs a multi-layered security approach that includes:
Quality Management.
YPrime upholds the highest standards in clinical research compliance, ensuring data integrity and participant safety. Our independent audits reinforce our commitment to excellence, while our quality assurance team proactively adapts to evolving regulations.

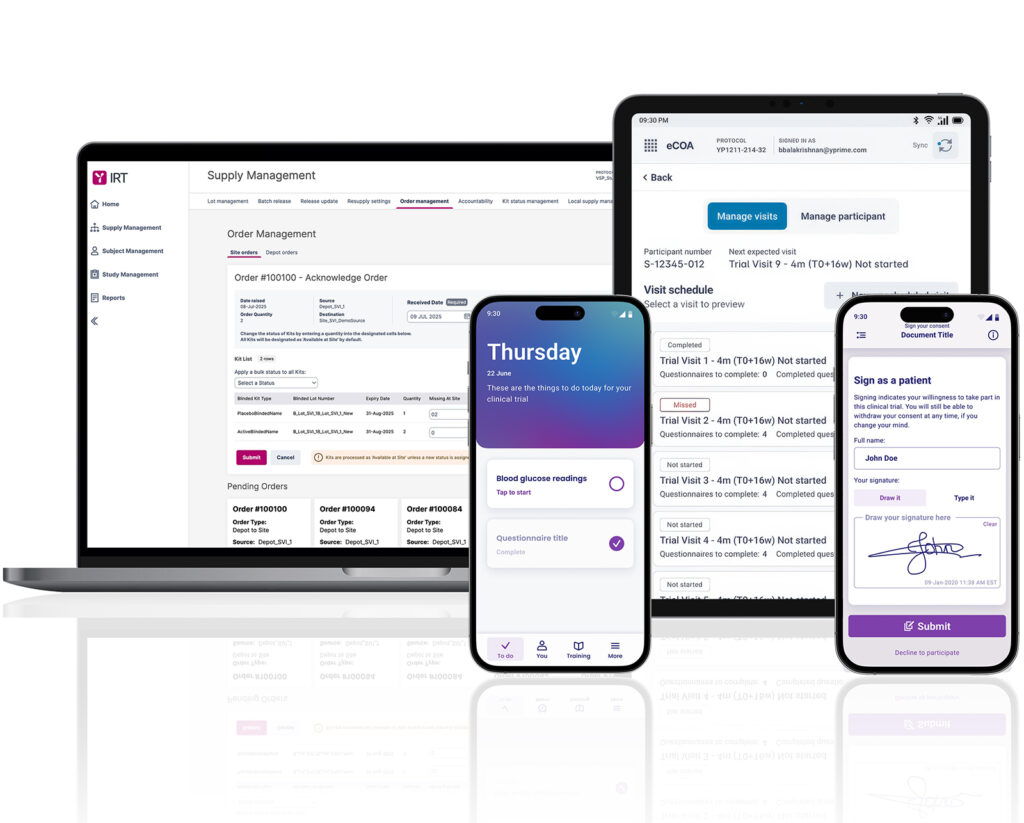
Technology Designed for Ease and Compliance.
YPrime’s eCOA, IRT, and eConsent platform provides seamless access through an intuitive, user-friendly interface. Our centralized documentation hub and responsive support team provide comprehensive resources for all clinical research stakeholders. We design our solutions with user experience in mind, incorporating feedback from sponsors, CROs, and sites to create tools that streamline workflows and reduce complexity in clinical trials.
Client Audit Program.
YPrime maintains an open-door policy for client audits of our eCOA, IRT, and eConsent. Our streamlined process makes it easy to:


Privacy Policy.
Protecting your privacy is integral to everything we do. Our comprehensive privacy policy outlines how we collect, use, and secure your data in compliance with:
By implementing privacy-by-design principles, YPrime ensures that data protection is built into every solution—not added as an afterthought.
Your Trust is Our Foundation.
At YPrime, trust isn’t just a commitment—it’s built into everything we do. From robust security protocols to transparent documentation, our approach to compliance and quality ensures your clinical trials run seamlessly.
Experience the confidence of working with a partner who prioritizes trust at every step.
Connect with an expert today.

